Gov. Janet Mills announced Monday that the federal government will nearly double Maine’s ration of a lifesaving drug that helps keep high-risk individuals infected with COVID-19 from winding up in intensive care.
Maine’s share of the monoclonal antibody sotrovimab will be 120 courses instead of the 66 Maine was scheduled to receive this week, Mills’ office said, following the governor’s call over the weekend to the White House’s coronavirus response coordinator, Jeff Zients. Mills called Zients “to make him aware that Maine was not receiving an allocation commensurate to the prevalence of the virus,” the governor’s office said.
The development came one day after a Portland Press Herald story revealed Maine is receiving a far lower share of the sotrovimab because a key metric used to allocate the life-saving medicine – per capita new case counts – has understated the prevalence of COVID-19 in Maine. Maine’s daily case counts have been artificially low because some 46,000 positive test results have yet to be added to official numbers by the Maine Center for Disease Control and Prevention.
The backlog of tests – which the Maine CDC screens to remove any duplications or residents of other states before adding to the official confirmed case tally – is far higher on a per capita basis than those in three other states where backlogs have made news this month.
On Thursday, Maine CDC Director Dr. Nirav Shah argued that there wasn’t a link between the case confirmation backlog and Maine’s reduced allocation, even though new per capita case counts are one of two components the federal Department of Health and Human Services says it uses for allocating the drug to states. On Friday, his spokesman, Robert Long, asserted that the U.S. DHHS was not allocating the drug via this formula.
But the governor’s office statement Monday did not make this argument, stating that the Maine Department of Health and Human Services and the Maine CDC had repeatedly made it clear to federal authorities “that Maine, along with other states, was confronting a backlog of cases that could lead to an artificially low distribution of monoclonal antibodies and raising questions about the distribution formula’s equity.”

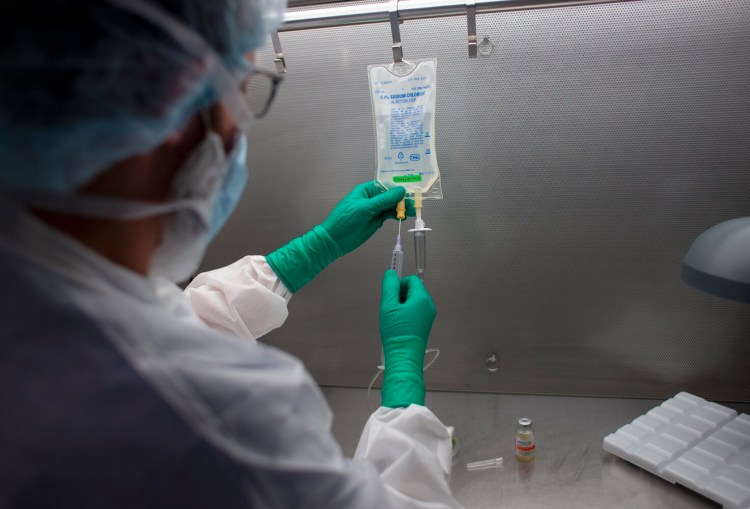
Dan Pelletier, a pharmacy technician specialist at Maine Medical Center, demonstrates the process of preparing antibodies used to treat COVID-19 patients, in September 2021. Derek Davis/Staff Photographer
FUTURE ALLOCATIONS UNCLEAR
Mills’ office said it was not clear if the increased allocation announced Monday would only be for this week or if it would be an ongoing increase. “But the administration is pleased that the federal government has made this change at a time when its own experts, including Dr. (Anthony) Fauci, are saying that case counts should become less of a focus,” the statement said.
Mills’ office said it would continue to work closely with the White House and other federal officials on Maine’s allocations and “will continue to improve its reporting on COVID-19 prevalence and trends in Maine.”
The U.S. DHHS has not responded to repeated requests for information about how the drug has been allocated, including an interview request sent to Deputy Assistant Secretary for Public Affairs for Public Health Bill Hall. Mills spokesperson Lindsay Crete said the department had not given them any additional information on this point.
For the past week, Maine’s official per capita new confirmed case numbers have been the lowest in the country. But those numbers didn’t account for the 46,000 backlogged positive tests. Because Maine’s official data has it as the safest place in the country for new cases, the state’s allocation of treatments has been low in recent weeks with not nearly enough to go around to all the people who would normally be prescribed it.
According to the U.S. DHHS website, it rations the drug out to states each week based on their shares of the nation’s COVID-19 hospitalizations and new cases over the previous seven days. This is consistent with what recent allocation data shows.
Last week Maine and New Hampshire had wildly different allocations even though they have nearly the same population (about 1.3 million) and on Friday had exactly the same seven-day per capita rate of hospitalizations (33 per 100,000 people). Maine received 72 doses of sotrovimab. New Hampshire received 180.
Vermont has less than half as many people as Maine and a significantly lower per capita hospitalization rate of 20 per 100,000 people, but received 96 doses.
Maine’s backlog in confirming positive cases stems in part from not yet having stopped performing individual case investigations as some other states have, said Long, the Maine CDC spokesman.
INVESTIGATIONS TAKE TIME
He said the process is labor intensive, requiring 3 to 5 minutes of data entry for each case – and that the agency has seen a dramatic increase in positive tests being submitted this month. The state has received more than 2,000 positive tests a day since Jan. 3 and received more than 3,000 positive tests on six days in the past two weeks, according to data posted on the CDC website.
There have been only 1,200-1,800 officially reported new cases a day during this period.
Long said the case investigations require the agency to determine and record additional information during the confirmation process: whether the individual is hospitalized, vaccinated, connected with an outbreak, pregnant, or previously had COVID-19.
“This process in part is why other jurisdictions across the country have scaled back or altogether stopped conducting case investigations,” Long said via email. “Those that stopped case investigations have been able to move toward automation.”
“Because of omicron’s sustained strain on our case reporting system, Maine CDC is evaluating potential changes to its case investigation and contact tracing processes in an effort to address the backlog and improve its reporting on COVID-19 prevalence and trends in Maine,” he added.
Monocolonal antibodies, laboratory-produced versions of a natural protein, are designed to attack the coronavirus that causes COVID-19. They are generally prescribed to COVID-19 patients ages 60 and older and younger people with high-risk conditions, such as obesity, diabetes or conditions that suppress the immune system, shortly after they test positive, to reduce the chance they become acutely sick. Sotrovimab is the only type of monoclonal antibody known to be effective against the omicron variant.
In December, the U.S. Food and Drug Administration approved the use of another treatment, an antiviral pill from Pfizer called Paxlovid, that also appears effective at fighting omicron cases. But its supplies also have been severely depleted in Maine and nationally. Another therapy, the antiviral remdesivir, is not in short supply but is harder to administer to outpatients because it requires three injections on three consecutive days, which can be difficult for people with work, parenting or transportation challenges.
Send questions/comments to the editors.



Comments are no longer available on this story